Abstract
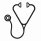
Background: Elderly lymphoma patients may present complex disease- and patient-related features that influence choice of therapy and outcomes. Recent US Lymphocare data suggest elderly patients are most commonly treated with watchful waiting or single-agent rituximab, unique from younger subgroups, and found no difference in outcomes by treatment groups-- but comorbidity was not studied. (Nabhan 2015) Comorbidity, as measured by the Charlson Comorbidity Index (CCI) with a cutoff of >1, has shown prognostic relevance in subsets of young FL patients and DLBCL, but has not been studied exclusively in elderly FL patients.
We aimed to describe patient features, comorbidity use of PET staging, management choices, and the impact of "polypharmacy" on outcomes of FL patients aged 70 or older. We specifically sought to address whether the CCI influences management choice or outcomes in this increasingly common subgroup of patients.
Methods: Patients with follicular lymphoma seen at the University of Washington/Seattle Cancer Care Alliance from 2008-2016 were identified through evaluation of ICD9/10 diagnosis codes. CCI was calculated, excluding lymphoma, for all patients who were divided into low CCI (CCI score of 0-1) or high CCI (≥2).
Information on clinical presentation (grade/stage), CCI, number of medications (prescription, herbal, and vitamin), and management details were collected. "Polypharmacy" was defined as #meds above median. Kaplan-Meier estimates of time to treatment, progression-free survival (PFS), and overall survival (OS) generated and subject to univariate /multivariate analysis (JMP 11.0/SAS) based on CCI score, FLIPI, grade, use of PET staging, and polypharmacy.
Results: 128 patients over age 70 were found, and 57 had adequate information and at least 3 months of follow-up from diagnosis; these were analyzed in detail.
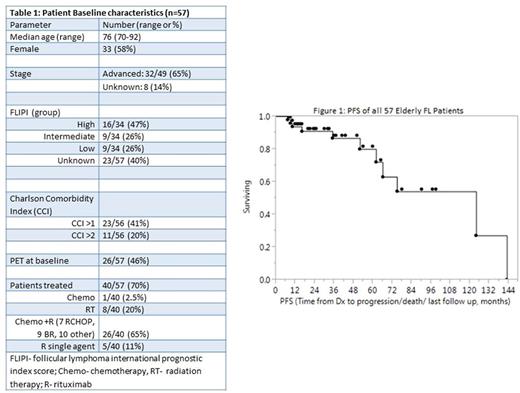
Patient characteristics are described in table 1. Median follow-up was 32 months. Most patients (34/57, 61%) had a CCI score of >1 and the median number of medications used was 8, including prescriptions, supplements, and vitamins. Of 57 patients, 26 (46%) underwent baseline PET imaging. 17 patients were observed initially, and the remaining 40 received either RT, R, or chemo/R.
The median time from diagnosis to initiation of therapy was 5 months based on Kaplan-Meier analysis. Time to treatment was not affected by CCI>1, use of PET in staging, or most clinical features. Only FL grade 3, more than 4 nodal sites, and high risk FLIPI influenced time to initiation of treatment.
Median PFS was over 5 years but continual relapses occurred with prolonged follow-up (Figure 1). PFS was unaffected by CCI>1, polypharmacy, or clinical features. Eight deaths occurred, though only 3 due to lymphoma. Analyzed against FLIPI, gender, and grade, only Hgb <12 predicted poorer OS (multivariate p=.007).
Conclusions
In this cohort of 57 elderly FL patients evaluated at a tertiary care center, relatively high rates of PFS and OS were observed. While follow-up for this dataset is only 32 months, and management decisions were non-standardized, neither CCI>1 nor presence of "polypharmacy" predicted time to initiation of therapy. On the other hand, high FLIPI score, grade 3, and high tumor burden /5 or more nodal sites predicted earlier time to treatment. Only presence of anemia was associated with worse OS. This data suggests that presently, disease-related factors remain a prime consideration for managing elderly FL patients in practice. Additionally, the CCI did not impact PFS or OS in elderly FL patients. Improved models for discriminating between comorbidities and tumor-related prognostic features for elderly FL patients are needed, to guide management of this growing population.
Shadman: Celgene: Research Funding; Merck: Research Funding; Acerta Pharma: Research Funding; AbbVie: Other: advisory board; PLEXXIKON: Research Funding; Emergent: Research Funding; Pharmacyclics: Other: advisory board, Research Funding; Gilead: Research Funding; Genentech: Consultancy, Research Funding; TG Therapeutics: Research Funding. Shustov: Spectrum Pharmaceuticals: Consultancy, Research Funding; Celgene: Other: Publication assistance. Smith: Janssen: Research Funding; Portola Pharmaceuticals: Research Funding; Acerta: Research Funding; Genentech: Research Funding; Merck: Research Funding; Dohme Corp: Research Funding; Sharp: Research Funding; Pharmacyclics LLC, an AbbVie Company: Research Funding; Seattle Genetics: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract